Abstract
Capogrosso, Milekovic, Borton et. al.
Spinal cord injury disrupts the communication between the brain and the spinal circuits that orchestrate movement. To bypass the lesion, brain-computer interfaces have directly linked cortical activity to electrical stimulation of muscles, and have thus restored grasping abilities after hand paralysis. Theoretically, this strategy could also restore control over leg muscle activity for walking. However, replicating the complex sequence of individual muscle activation patterns underlying natural and adaptive locomotor movements poses formidable conceptual and technological challenges. Recently, it was shown in rats that epidural electrical stimulation of the lumbar spinal cord can reproduce the natural activation of synergistic muscle groups producing locomotion. Here we interface leg motor cortex activity with epidural electrical stimulation protocols to establish a brain-spine interface that alleviated gait deficits after a spinal cord injury in non-human primates. Rhesus monkeys (Macaca mulatta) were implanted with an intracortical microelectrode array in the leg area of the motor cortex and with a spinal cord stimulation system composed of a spatially selective epidural implant and a pulse generator with real-time triggering capabilities. We designed and implemented wireless control systems that linked online neural decoding of extension and flexion motor states with stimulation protocols promoting these movements. These systems allowed the monkeys to behave freely without any restrictions or constraining tethered electronics. After validation of the brain-spine interface in intact (uninjured) monkeys, we performed a unilateral corticospinal tract lesion at the thoracic level. As early as six days post-injury and without prior training of the monkeys, the brain-spine interface restored weight-bearing locomotion of the paralysed leg on a treadmill and overground. The implantable components integrated in the brain-spine interface have all been approved for investigational applications in similar human research, suggesting a practical translational pathway for proof-of-concept studies in people with spinal cord injury.
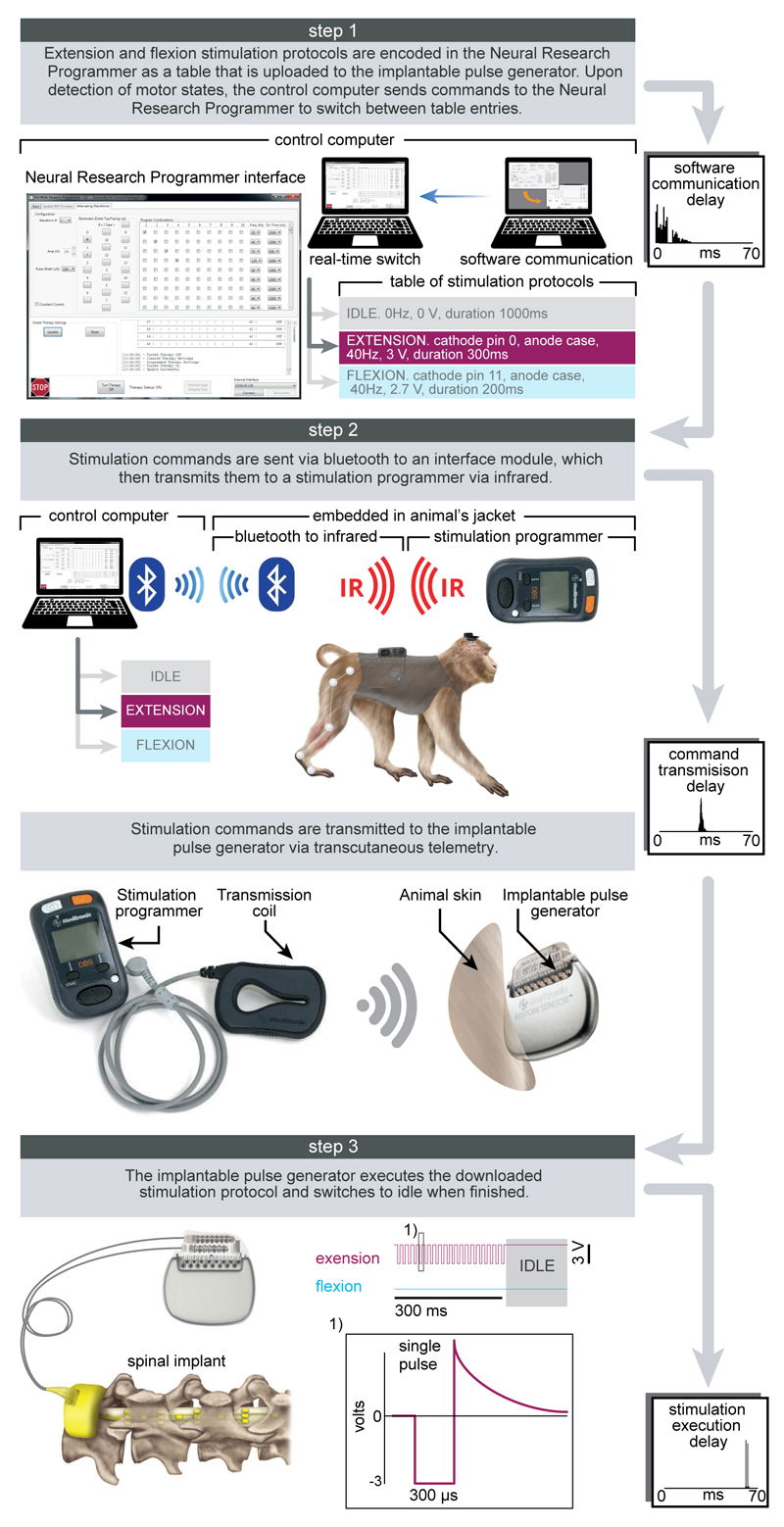
Protocols and technology of the spinal cord stimulation system.
Step 1: A Neural Research Programmer interface (screen snapshot) encodes stimulation protocols that are pre-programmed into a table uploaded to the implantable pulse generator. Each row of this table corresponds to a specific electrode configuration (cathodes and anodes) and stimulation features (amplitude, frequency, pulse width and duration of stimulation). During experiments, the control computer selects the rows to be executed. The plot reports the distribution of temporal delays introduced by the communication between the decoder and the Neural Research Programmer (n = 5000). Step 2: stimulation commands are transmitted to the implantable pulse generator. Commands are first broadcasted via Bluetooth to a module that converts them into infrared signals transferred to the stimulation programmer device. The Bluetooth to infrared module and the stimulation programmer were embedded into a jacket worn by the monkeys during the experiments. The stimulation programmer transmitted the stimulation commands into the implantable pulse generator via induction telemetry. The antenna was placed under the jacket, in contact with the skin and aligned to the implantable pulse generator. The plot reports the distribution of delays needed to transmit the stimulation commands from the Neural Research Programmer to the implantable pulse generator. Step 3: The implantable pulse generator executed the selected stimulation protocols. After execution of the stimulation command, the implantable pulse generator switched to idle mode. The shape of a single charge balanced cathodic pulse is shown in the inset (1). The plot reports the distribution of time delays required to execute a single stimulation command by the implantable pulse generator.
PMID: 27830790 PMCID: PMC5108412 DOI: 10.1038/nature20118





